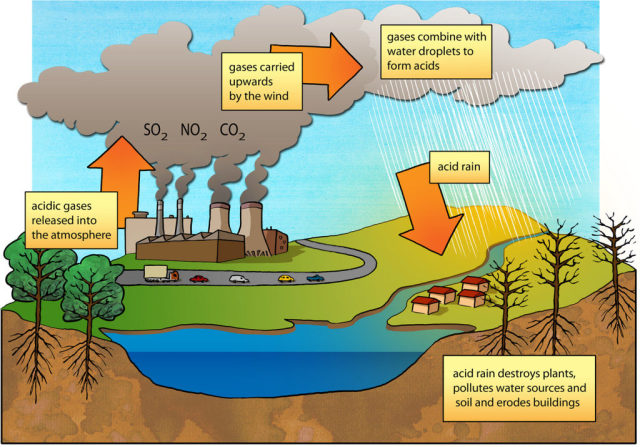
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions . It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Some governments have made efforts since the 1970s[1] to reduce the release of sulfur dioxide and nitrogen oxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes, and sulfur dioxide is produced by volcanic eruptions. Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic life-forms, causing paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and statues as well as having impacts on human health.
The principal natural phenomena that contribute acid-producing gases to the atmosphere are emissions from volcanoes. Acid-producing gasses are also created by biological processes that occur on the land, in wetlands, and in the oceans. The major biological source of sulfur compounds is dimethyl sulfide.
The principal cause of acid rain is sulfur and nitrogen compounds from human sources, such as electricity generation, animal agriculture, factories, and motor vehicles. Electrical power generation using coal is among the greatest contributors to gaseous pollution responsible for acidic rain. The gases can be carried hundreds of kilometers in the atmosphere before they are converted to acids and deposited. In the past, factories had short funnels to let out smoke but this caused many problems locally; thus, factories now have taller smoke funnels. However, dispersal from these taller stacks causes pollutants to be carried farther, causing widespread ecological damage.